Multiple Sclerosis
An unpredictable disease of the central nervous system, multiple sclerosis (MS) can range from relatively benign to somewhat disabling to devastating, as communication between the brain and other parts of the body is disrupted. Many investigators believe MS to be an autoimmune disease — one in which the body, through its immune system, launches a defensive attack against its own tissues. In the case of MS, it is the nerve-insulating myelin that comes under assault. Such assaults may be linked to an unknown environmental trigger, perhaps a virus.
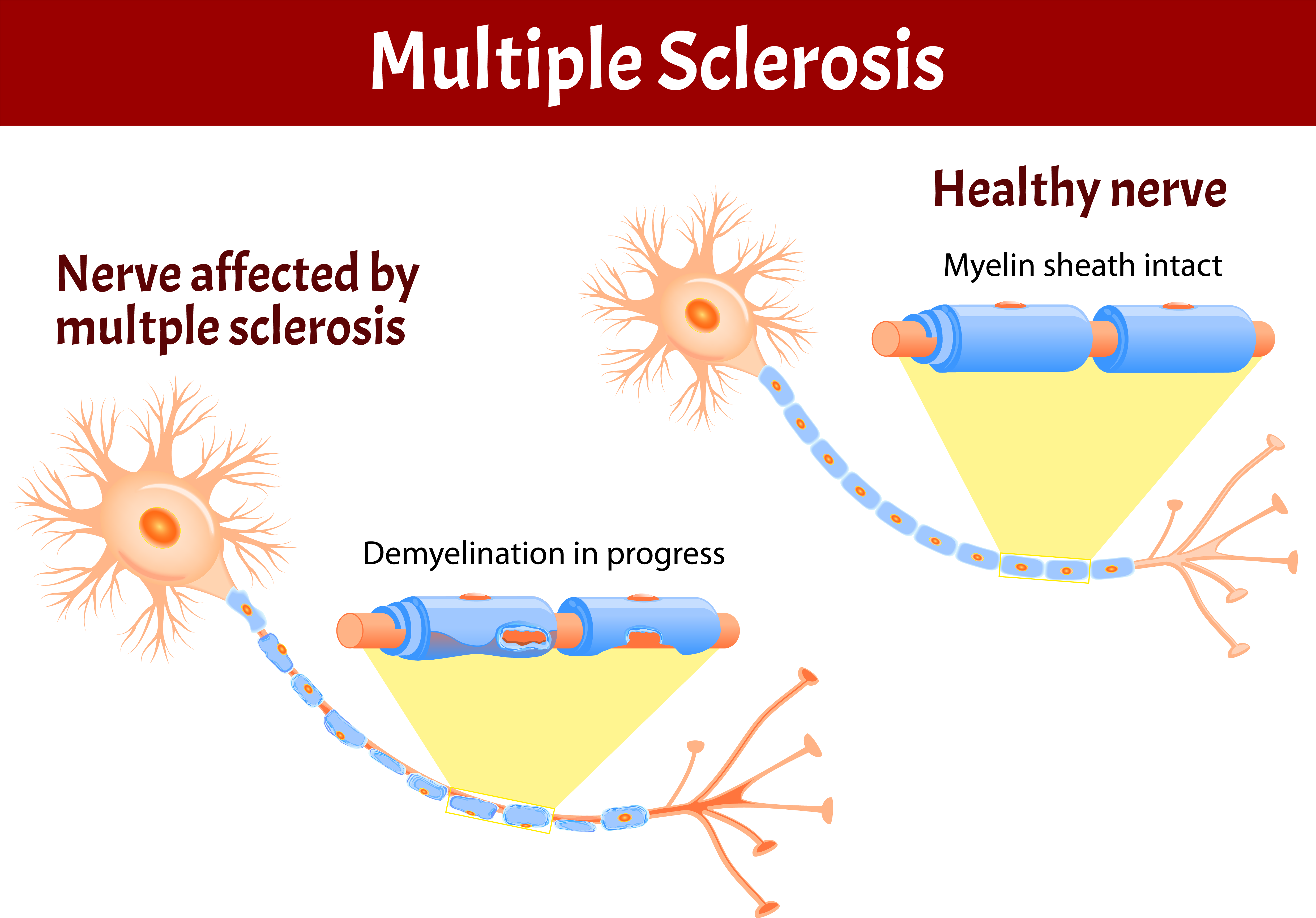
Most people experience their first symptoms of MS between the ages of 20 and 40; the initial symptom of MS is often blurred or double vision, red-green color distortion, or even blindness in one eye. Most MS patients experience muscle weakness in their extremities and difficulty with coordination and balance. These symptoms may be severe enough to impair walking or even standing. In the worst cases, MS can produce partial or complete paralysis. Most people with MS also exhibit paresthesias, transitory abnormal sensory feelings such as numbness, prickling, or “pins and needles” sensations. Some may also experience pain. Speech impediments, tremors, and dizziness are other frequent complaints. Occasionally, people with MS have hearing loss. Approximately half of all people with MS experience cognitive impairments such as difficulties with concentration, attention, memory, and poor judgment, but such symptoms are usually mild and are frequently overlooked. Depression is another common feature of MS.
Currently there is no cure for MS. Many individuals do well with no therapy at all, especially since many medications have serious side effects and some carry significant risks. However, three forms of beta interferon (Avonex, Betaseron, and Rebif) have now been approved by the Food and Drug Administration for treatment of relapsing-remitting MS. The FDA has also approved ocrelizumab (brand name Ocrevus) to treat adults with relapsing forms of MS and primary progressive MS. Beta interferon has been shown to reduce the number of exacerbations and may slow the progression of physical disability. When attacks do occur, they tend to be shorter and less severe. The FDA also has approved a synthetic form of myelin basic protein, called copolymer I (Copaxone), for the treatment of relapsing-remitting MS. Copolymer I has few side effects, and studies indicate that the agent can reduce the relapse rate by almost one third. Other FDA approved drugs to treat relapsing forms of MS in adults include teriflunomide and dimethyl fumarate. An immunosuppressant treatment, Novantrone (mitoxantrone), is approved by the FDA for the treatment of advanced or chronic MS. The FDA has also approved dalfampridine (Ampyra) to improve walking in individuals with MS.
One monoclonal antibody, natalizumab (Tysabri), was shown in clinical trials to significantly reduce the frequency of attacks in people with relapsing forms of MS and was approved for marketing by the U.S. Food and Drug Administration (FDA) in 2004. However, in 2005 the drug’s manufacturer voluntarily suspended marketing of the drug after several reports of significant adverse events. In 2006, the FDA again approved sale of the drug for MS but under strict treatment guidelines involving infusion centers where patients can be monitored by specially trained physicians.
While steroids do not affect the course of MS over time, they can reduce the duration and severity of attacks in some patients. Spasticity, which can occur either as a sustained stiffness caused by increased muscle tone or as spasms that come and go, is usually treated with muscle relaxants and tranquilizers such as baclofen, tizanidine, diazepam, clonazepam, and dantrolene. Physical therapy and exercise can help preserve remaining function, and patients may find that various aids — such as foot braces, canes, and walkers — can help them remain independent and mobile. Avoiding excessive activity and avoiding heat are probably the most important measures patients can take to counter physiological fatigue. If psychological symptoms of fatigue such as depression or apathy are evident, antidepressant medications may help. Other drugs that may reduce fatigue in some, but not all, patients include amantadine (Symmetrel), pemoline (Cylert), and the still-experimental drug aminopyridine. Although improvement of optic symptoms usually occurs even without treatment, a short course of treatment with intravenous methylprednisolone (Solu-Medrol) followed by treatment with oral steroids is sometimes used.
A physician may diagnose MS in some patients soon after the onset of the illness. In others, however, doctors may not be able to readily identify the cause of the symptoms, leading to years of uncertainty and multiple diagnoses punctuated by baffling symptoms that mysteriously wax and wane. The vast majority of patients are mildly affected, but in the worst cases, MS can render a person unable to write, speak, or walk. MS is a disease with a natural tendency to remit spontaneously, for which there is no universally effective treatment.
Neurological Diagnostic Tests and Procedures
Fact sheet on neurological diagnosis and testing, prepared by the National Institute of Neurological Disorders and Stroke (NINDS).
Multiple Sclerosis: Hope Through Research
Multiple Sclerosis (MS) information sheet compiled by the National Institute of Neurological Disorders and Stroke (NINDS).
Esclerosis Múltiple: Esperanza en la Investigacion
Patient Organizations
Accelerated Cure Project for Multiple Sclerosis
460 Totten Pond Rd. Suite 420
Waltham, MA 02451
info@acceleratedcure.org
www.acceleratedcure.org
(781) 487-0008
Myelin Repair Foundation
18809 Cox Avenue
Suite 190
Saratoga, CA 95070
info@myelinrepair.org
www.myelinrepair.org
(408) 871-2410
Well Spouse Association
63 West Main Street
Suite H
Freehold, NJ 07728
info@wellspouse.org
www.wellspouse.org
(800) 838-0879
(732) 577-8899
American Autoimmune Related Diseases Association
22100 Gratiot Avenue
Eastpointe, MI 48021-2227
aarda@aarda.org
www.aarda.org
(586) 776-3900
(800) 598-4668
National Ataxia Foundation (NAF)
600 Highway 169 South
Suite 1725
Minneapolis, MN 55426
naf@ataxia.org
www.ataxia.org
(763) 553-0020
Osers
Office of Special Education and Rehabilitative Services
550 12th Street, SW, Rm. 5133
Washington, DC 20202-2550
www.ed.gov/about/offices/list/osers
(202) 245-7307
(202) 205-5637 (TTD)
National Multiple Sclerosis Society
733 Third Avenue
3rd Floor
New York, NY 10017-3288
ContactUsNMSS@nmss.org
www.nationalmssociety.org
(800) 344-4867
Multiple Sclerosis Association of America
375 Kings Highway North
Cherry Hill, NJ 08034
webmaster@msassociation.org
www.mymsaa.org
(856) 488-4500
(800) 532-7667
National Rehabilitation Information Center (NARIC)
8400 Corporate Drive
Suite 500
Landover, MD 20785
naricinfo@heitechservices.com
www.naric.com
(301) 459-5900
(800) 346-2742
(301) 459-5984 (TTY)
Multiple Sclerosis Foundation
6520 North Andrews Avenue
Ft. Lauderdale, FL 33309-2130
support@msfocus.org
www.msfocus.org
(954) 776-6805
(888) 673-6287
Paralyzed Veterans of America (PVA)
801 18th Street, NW
Washington, DC 20006-3517
info@pva.org
www.pva.org
(202) 872-1300
(800) 555-9140
Prepared by:
Office of Communications and Public Liaison
National Institute of Neurological Disorders and Stroke
National Institutes of Health
Bethesda, MD 20892
NINDS health-related material is provided for information purposes only and does not necessarily represent endorsement by or an official position of the National Institute of Neurological Disorders and Stroke or any other Federal agency. Advice on the treatment or care of an individual patient should be obtained through consultation with a physician who has examined that patient or is familiar with that patient’s medical history.
All NINDS-prepared information is in the public domain and may be freely copied. Credit to the NINDS or the NIH is appreciated Where to find on the internet: https://www.ninds.nih.gov/Disorders/All-Disorders/Multiple-Sclerosis-Information-Page
Our Mission

Founded in 2005, the APS Foundation of America, Inc. is dedicated to fostering and facilitating joint efforts in the areas of education, public awareness, research, and patient services for Antiphospholipid Syndrome (APS) in an effective and ethical manner.
Information Sheets
DISCLAIMER: The APS Foundation of America, Inc. website is not intended to replace standard doctor-patient visits, physical examination, and medical testing. Information given to members is only an opinion. All information should be confirmed with your doctor. Always seek a trained physician's advice before seeking any new treatment regarding your medical diagnosis or condition. Any information received from the APS Foundation of America, Inc. website is not intended to diagnose, treat, or cure. This site is for informational purposes only. Please note that we will list all donors' or purchasers' names on the donor page of our foundation site. If you do not want your name listed, please get in touch with us to opt-out. If you think you may have a medical emergency, call your doctor or 911 immediately.











